A) HO ¾
B) CH3NH ¾
C) CH3O ¾
D) (CH3) 3N+¾
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why is sulfuric acid used in aromatic nitration?
A) To keep the reaction from getting too basic
B) To form the active electrophile NO2+
C) To protonate the aromatic ring
D) To keep the reaction from getting too acidic
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the reactive intermediate formed in the elimination-addition mechanism of nucleophilic aromatic substitution?
A) Carbocation
B) Radical
C) Benzyne
D) Carbanion
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is a major problem with Friedel-Crafts alkylation?
A) It requires high temperatures.
B) The conditions are too acidic.
C) The starting material is frequently over-alkylated.
D) The products coordinate with the aluminum chloride.
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How can polyalkylation be minimized in Friedel-Crafts alkylation?
A) Use a large excess of alkyl halide relative to the aromatic compound.
B) Use a large excess of benzene relative to the alkyl halide.
C) Use an alkyl halide without a Lewis acid catalyst.
D) Use a large excess of the Lewis acid catalyst.
F) None of the above
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Consider the tetracyclic aromatic compound drawn below,with rings labelled as I,II,III,and IV.Which of the four rings is least reactive in electrophilic aromatic substitution? 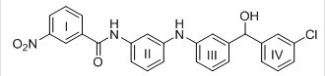
A) I
B) II
C) III
D) IV
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What are the two effects that have to be considered to determine the influence a substituent will have on electrophilic aromatic substitution?
A) Steric and electronic
B) Inductive and steric
C) Inductive and resonance
D) Resonance and electronic
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is (are) the product(s) of the following reaction? 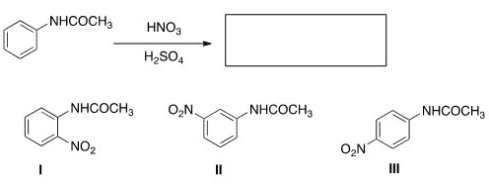
A) Only I
B) Only II
C) Only III
D) Only I and III
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What are the product(s) of the following reaction? 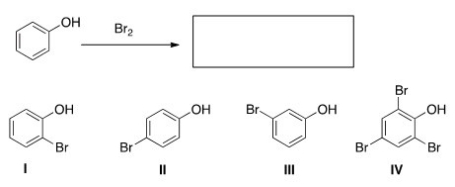
A) Only I and II
B) Only I and III
C) Only II and III
D) Only IV
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What are the product(s) of the following reaction? 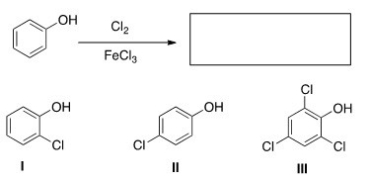
A) Only I
B) Only II
C) Only I and II
D) Only III
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the following compounds in order of decreasing reactivity in electrophilic aromatic substitution. 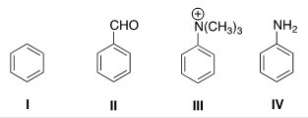
A) II > IV > I > III
B) II > III > IV > I
C) IV > II > I > III
D) IV > I > II > III
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the driving force for losing a proton as the last step in electrophilic aromatic substitution?
A) To neutralize the base that is present
B) To make room for the electrophile
C) To make the ring more reactive
D) To rearomatize the ring system
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why is the nitro group a meta director?
A) Because it is sterically very large
B) Because it adds electron density to the meta position,thus activating it
C) Because it stabilizes the intermediate cation
D) Because it removes more electron density from the ortho and para positions than the meta position,thus deactivating the meta position less
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the following activating groups in order of decreasing strength of activation,listing the most activating first. 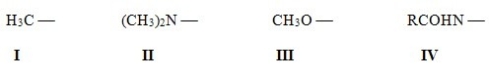
A) IV > II > III > I
B) II > III > IV > I
C) III > IV > II > I
D) II > IV > III > I
F) A) and D)
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
What reagents would be necessary to produce the following product from benzene? 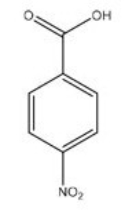
A) CH3Cl,AlCl3 2.HNO3,H2SO4 3.KMnO4
B) HNO3,H2SO4 2.CH3Cl,AlCl3 3.KMnO4
C) KMnO4 2.CH3Cl,AlCl3 3.HNO3,H2SO4
D) KMnO4 2.HNO3,H2SO4 3.CH3Cl,AlCl3
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which aryl fluoride reacts the fastest with NaOH? 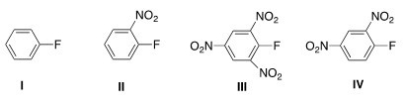
A) I
B) II
C) III
D) IV
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the major organic product obtained from the following reaction? 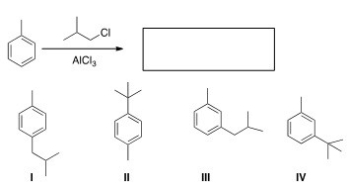
A) I
B) II
C) III
D) IV
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the best choice of reagent to bring about the following transformation? ![What is the best choice of reagent to bring about the following transformation? A) [1] LiAlH<sub>4</sub>; [2]H<sub>2</sub>O B) Zn (Hg) ,HCl C) NH<sub>3</sub>,NaOH D) H<sub>2</sub>,Pd-C](https://d2lvgg3v3hfg70.cloudfront.net/TB7662/11eac43c_f7bd_e34a_a9af_838f0b816626_TB7662_00.jpg)
A) [1] LiAlH4; [2]H2O
B) Zn (Hg) ,HCl
C) NH3,NaOH
D) H2,Pd-C
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the major organic product obtained from the following reaction? 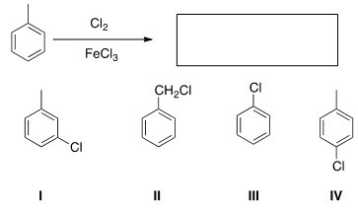
A) I
B) II
C) III
D) IV
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the major product of electrophilic addition of HBr to the following alkene? 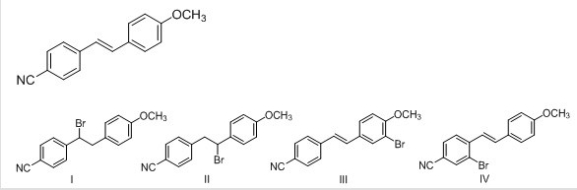
A) I
B) II
C) III
D) IV
F) B) and C)
Correct Answer

verified
B
Correct Answer
verified
Showing 1 - 20 of 60
Related Exams