A) ![]()
B) ![]()
C) ![]()
D) ![]()
F) All of the above
Correct Answer

verified
Correct Answer
verified
True/False
The compound below contains no polar bonds. 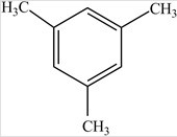
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the bond angle associated with a tetrahedral shape?
A) 109.5º
B) 180º
C) 120º
D) 90º
E) 60º
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the correct molecular formula for the skeletal structure given below? 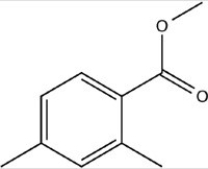
A) C11H16O2
B) C12H12O2
C) C10H12O2
D) C10H15O2
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which compound is an ether?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which structure properly indicates the order of attachment of atoms as represented by the condensed structure CH3CHBrCH2CH(CH3) 2?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Short Answer
Vitamin E (structure shown)is a (fat/water)________-soluble vitamin. 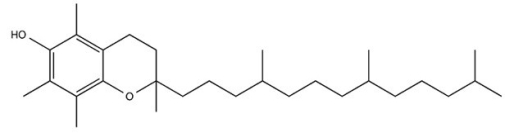
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The compound below is classified as what type of compound? 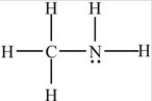
A) Ether
B) Amine
C) Amide
D) Aldehyde
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which structure is not possible?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
F) All of the above
Correct Answer

verified
Correct Answer
verified
Short Answer
In organic compounds,any atom that is not carbon or hydrogen is called a/an ________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the shape around each carbon atom in the structure shown below? 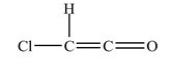
A) The shape around each carbon atom is tetrahedral.
B) The shape around each carbon atom is bent.
C) The shape around the left carbon atom is tetrahedral and bent around the right carbon atom.
D) The shape around the left carbon atom is trigonal planar and bent around the right carbon atom.
E) The shape around the left carbon atom is trigonal planar and linear around the right carbon atom.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many covalent bonds does nitrogen typically form in organic compounds?
A) 1
B) 3
C) 5
D) 8
F) A) and B)
Correct Answer

verified
Correct Answer
verified
True/False
In order to complete the structure of the environmental toxin dioxin shown below,four H atoms and two lone pairs need to be added. 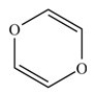
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which formula represents an inorganic compound?
A) CH3CO2CH2CH3
B) CH3NHCH2CH3
C) ClCCCl
D) CaCl2
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which skeletal structure represents the compound with the following condensed structure: (CH3) 3CCH2OH?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which structure has all of the hydrogens and lone pairs correctly added to the compound shown below? 
A) ![]()
B) ![]()
C) ![]()
D) ![]()
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What are the bond angles in the structure shown below? 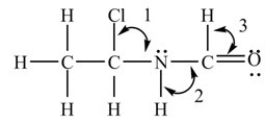
A) Angle 1 = 109.5°,Angle 2 = 109.5°,and Angle 3 = 109.5°
B) Angle 1 = 109.5°,Angle 2 = 120°,and Angle 3 = 120°
C) Angle 1 = 109.5°,Angle 2 = 90°,and Angle 3 = ~109.5°
D) Angle 1 = 109.5°,Angle 2 = ~109.5°,and Angle 3 = 120°
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Short Answer
In terms of type of compound,the compound shown below is classified as a/an ________. 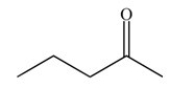
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which compound is an aldehyde?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
F) None of the above
Correct Answer

verified
Correct Answer
verified
True/False
A functional group is an atom or a group of atoms with characteristic chemical and physical properties.
B) False
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 123
Related Exams