A) number of carbon, hydrogen, and oxygen atoms
B) types of carbon, hydrogen, and oxygen atoms
C) arrangement of carbon, hydrogen, and oxygen atoms
D) number of oxygen atoms joined to carbon atoms by double covalent bonds
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the figures to answer the question.
A.
C.
B.
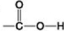 D.
Which of the groups is an acidic functional group that can dissociate and release H⁺ into a solution?
D.
Which of the groups is an acidic functional group that can dissociate and release H⁺ into a solution?
A) A
B) B
C) C
D) D
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The element present in all organic molecules is ________.
A) hydrogen
B) oxygen
C) carbon
D) nitrogen
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Differences among organisms are caused by differences in the ________.
A) elemental composition from organism to organism
B) types and relative amounts of organic molecules synthesized by each organism
C) sizes of the organic molecules in each organism
D) types of inorganic compounds present in each organism
F) B) and C)
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Use the figures to answer the question.
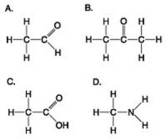 Which molecule shown can increase the concentration of hydrogen ions in a solution and is therefore an organic acid?
Which molecule shown can increase the concentration of hydrogen ions in a solution and is therefore an organic acid?
A) A
B) B
C) C
D) D
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Testosterone and estradiol are male and female sex hormones, respectively, in many vertebrates. In what way(s) do these molecules differ from each other? Testosterone and estradiol ________.
A) are structural isomers but have the same molecular formula
B) are cis-trans isomers but have the same molecular formula
C) have different functional groups attached to the same carbon skeleton
D) are enantiomers of the same organic molecule
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules is a part of ATP?
A) adenosine
B) cytosine
C) guanine
D) uracil
F) All of the above
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
Use the figures to answer the question.
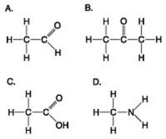 Which molecule(s) shown is (are) ionized in a cell?
Which molecule(s) shown is (are) ionized in a cell?
A) A
B) B and D
C) C and D
D) D
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the figures to answer the question.
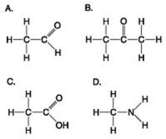 Which molecule shown has a carbonyl functional group in the form of an aldehyde?
Which molecule shown has a carbonyl functional group in the form of an aldehyde?
A) A
B) B
C) C
D) D
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements correctly describes cis-trans isomers?
A) They have variations in arrangement around a double bond.
B) They have an asymmetric carbon that makes them mirror images.
C) They have the same chemical properties.
D) They have different molecular formulas.
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The kind and number of bonds an atom can form depends on ________.
A) its atomic number
B) its electron configuration
C) its atomic mass
D) the number of particles in its nucleus
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following illustrations is not a structural isomer of an organic compound with the molecular formula C₆H₁₄? For clarity, only the carbon skeletons are shown; hydrogen atoms that would be attached to the carbons have been omitted.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
F) C) and D)
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
Compared to a hydrocarbon chain where all the carbon atoms are linked by single bonds, a hydrocarbon chain with the same number of carbon atoms but with one or more double bonds will ________.
A) be more flexible in structure
B) be more constrained in structure
C) be more polar
D) have more hydrogen atoms
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
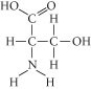 Which functional group is not present in this molecule?
Which functional group is not present in this molecule?
A) carboxyl
B) sulfhydryl
C) hydroxyl
D) amino
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following carbon molecules does not have the bond angle of 109.5°?
A) CH₄
B) C₂H₄
C) C₂H₆
D) C₃H₈
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is true?
A) ADP contains more energy than ATP.
B) Following hydrolysis, ATP can give off one phosphate, whereas ADP cannot.
C) ADP can have two positive charges.
D) ATP can have four negative charges.
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What determines whether a carbon atom's covalent bonds to other atoms are in a tetrahedral configuration or a planar configuration?
A) the presence or absence of bonds with oxygen atoms
B) the presence or absence of double bonds between the carbon atom and other atoms
C) the polarity of the covalent bonds between carbon and other atoms
D) the solvent in which the organic molecule is dissolved
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Visualize the structural formula of each of the following hydrocarbons. Which hydrocarbon has a double bond in its carbon skeleton?
A) C₃H₈
B) C₂H₆
C) C₂H₄
D) C₂H₂
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following figure to answer the question.
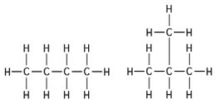 The two molecules shown in the figures are best described as ________.
The two molecules shown in the figures are best described as ________.
A) enantiomers
B) structural isomers
C) cis-trans isomers
D) chain length isomers
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the pairs of molecular structures shown depict enantiomers (enantiomeric forms) of the same molecule?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 58
Related Exams