A) 0.50
B) 0.25
C) 3.0 x 1023
D) 1.5 x 1023
E) 4.2 x 10-25
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is incorrect?
A) PH3 has 3 times as many hydrogen atoms as phosphorus atoms.
B) MgO has an equal number of magnesium ions and oxygen ions.
C) BaBr2 has half as many barium ions as bromide ions.
D) N2O5 has 2.5 times as many nitrogen atoms as oxygen atoms.
E) SF4 has 4 times as many fluorine atoms as sulfur atoms.
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the molarity of a solution consisting of 73.8 g NaCl in 1.50 L of solution?
A) 0.528 M
B) 1.19 M
C) 49.2 M
D) 1.26 M
E) 0.842 M
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many nitrogen atoms are present in 0.150 mol of Zn(NO3) 2?
A) 9.03 x 1022 atoms
B) 1.81 x 1023 atoms
C) 4.98 x 10-25 atoms
D) 0.300 atoms
E) 0.450 atoms
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The molecular formula of the molecules in the figure is: 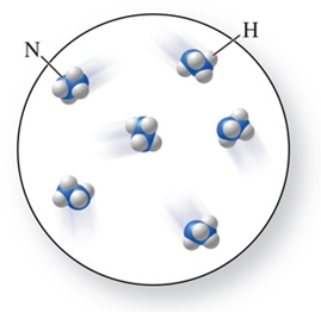
A) NH4
B) N2H4
C) NH3
D) N2H3
E) N4H2
G) C) and E)
Correct Answer

verified
Correct Answer
verified
True/False
The substance in the figure with the most atoms per mole is NaCl. 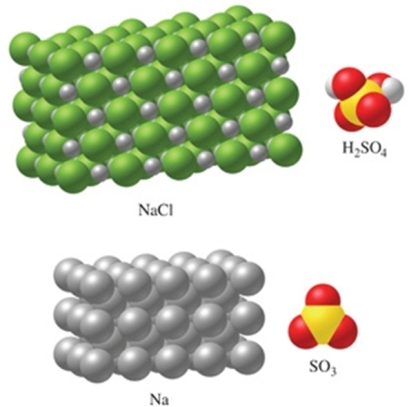
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements regarding empirical formulas, molecular formulas, and percent composition is correct?
A) The compounds NO2 and N2O4 have the same empirical formula.
B) The compounds NO2 and N2O4 have the same molecular formula.
C) The empirical formula of H2O2 is H2O.
D) The empirical formula of N2O5 is NO2.5.
E) The compounds PCl3 and PCl5 would have the same percent composition.
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the molarity of a solution consisting of 60.0 g of NaOH in 1.50 L of solution.
A) 40.0 M
B) 1.00 M
C) 1.74 M
D) 1.50 M
E) 1.60 x 103 M
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many formula units are in 0.25 mole of Na2O?
A) 4.5 x 1023
B) 0.75
C) 1.5 x 1023
D) 4.2 x 10-25
E) 0.25
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If 100.0 mL of 0.426 M NaOH solution is diluted to 500.0 mL, what is the concentration of the diluted solution?
A) 0.0426 M
B) 0.0852 M
C) 0.117 M
D) 2.13 M
E) 8.52 x 103 M
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many oxygen atoms are present in 1.50 mol of Zn(NO3) 2?
A) 4.50 atoms
B) 9.00 atoms
C) 9.03 x 1023 atoms
D) 5.42 x 1024 atoms
E) 1.49 x 10-23 atoms
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the following elements in order from least to most number of moles of atoms in a 10.0 g sample: Sn, Si, Se, S
A) Sn < Se < S < Si
B) Si < S < Se < Sn
C) Si < S < Sn < Se
D) S < Si < Se < Sn
E) Se < Sn < S < Si
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many molecules of HCl are present in 0.250 mol of HCl?
A) 4.15 x 10-25 molecules
B) 1.51 x 1023 molecules
C) 2.41 x 1024 molecules
D) 9.11 molecules
E) 0.250 molecules
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following sets of formulas is correct for the molecules in the figure? 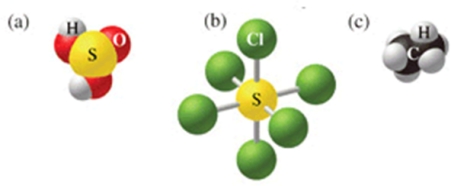
A) H2SO3, SCl5, C2H5
B) H2SO4, SCl5, C2H5
C) H2SO4, SCl6, C2H6
D) H2SO4, SCl6, CH6
E) H2SO3, SCl6, C2H6
G) D) and E)
Correct Answer

verified
Correct Answer
verified
True/False
The process of diluting a solution in the laboratory is much like preparing juice from a canned concentrate of the juice.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many formula units are in 2.0 moles of Fe(NO3) 2?
A) 1.2 x 1024
B) 3.6 x 1024
C) 1.1 x 1025
D) 2.0
E) 18
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is incorrect?
A) H2SO3 has 1.5 times as many oxygen atoms as hydrogen atoms.
B) Fe2(SO4) 3 has six times as many oxygen atoms as iron ions.
C) N2H4 has twice as many hydrogen atoms as nitrogen atoms.
D) MgF2 has half as many fluoride ions as magnesium ions.
E) Carbon dioxide has twice as many oxygen atoms as carbon atoms.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If 7.50 x 1024 molecules of a substance have a mass of 454 g, what is the molar mass of the substance?
A) 2.73 x 1026 g/mol
B) 3.41 x 1027 g/mol
C) 5.65 x 103 g/mol
D) 36.5 g/mol
E) 12.5 g/mol
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements regarding empirical formulas, molecular formulas, and percent composition is correct?
A) The empirical formula and molecular formula of a given compound are always the same.
B) Two compounds having different percent compositions of the same two elements are not the same compound.
C) The empirical formula and molecular formula of a given compound are never the same.
D) Data on the percent composition of the elements in a given compound is insufficient to determine the empirical formula of the compound.
E) The compound S2Cl2 would have the same empirical and molecular formulas.
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Methyl butyrate is a compound that is partially responsible for the flavor of apples.It consists of 58.80% C, 9.87% H, and 31.33% O.What is the empirical formula of methyl butyrate?
A) C6H10O3
B) C5H10O2
C) CH2O
D) C4.9H9.8O1.9
E) C4H9O2
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 111
Related Exams