A) 2.50 x 102 L
B) 2.50 x 102 mL
C) 36.0 mL
D) 36.0 L
E) 9.11 L
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many formula units are in a sample of rust, Fe2O3 that has a mass of 5.0 g?
A) 1.9 x 1025
B) 0.031
C) 1.9 x 1022
D) 0.070
E) 4.2 x 1022
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Butyric acid has a very unpleasant odor.It is present in some rotting foods, body odor and vomit.Butyric acid has a molar mass of 88.10 g/mol and consists of 54.53% C, 9.153% H, and 36.32% O.What is the molecular formula of butyric acid?
A) CH2O0.5
B) CH2O
C) CH4O
D) C2H4O
E) C4H8O2
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the number of moles of CaCO3 (calcium carbonate, or limestone) in a 20.0 g sample of this substance.
A) 2.00 x 103 moles
B) 0.200 mole
C) 0.294 mole
D) 1.36 x 103 moles
E) 1.20 x 103 moles
G) A) and E)
Correct Answer

verified
Correct Answer
verified
True/False
Molarity is defined as the moles of solute divided by the liters of solvent.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Barium sulfate is a compound used to assist in diagnosing medical problems through x-ray analysis and is 58.8% barium.What mass of barium is present in a 620 mg tablet of barium sulfate?
A) 81 mg
B) 230 mg
C) 360 mg
D) 1100 mg
E) 11 mg
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
4.Malachite is a green colored mineral that is 57.5% copper.What mass of copper is present in a 250.0 g sample of malachite?
A) 63.6 g
B) 127 g
C) 435 g
D) 36.5 g
E) 144 g
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A chemical reaction requires 3.50 moles of copper(II) nitrate, Cu(NO3) 2.What mass of copper(II) nitrate is needed?
A) 327 g
B) 93.6 g
C) 188 g
D) 656 g
E) 53.6 g
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is incorrect?
A) CH4 has ¼ as many carbon atoms as hydrogen atoms.
B) H2O has twice as many oxygen atoms as hydrogen atoms.
C) SO3 has three times as many oxygen atoms as sulfur atoms.
D) P4O10 has 2.5 times as many oxygen atoms as phosphorus atoms.
E) SrF2 has twice as many fluoride ions as strontium ions.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
True/False
To obtain 0.50 moles of potassium nitrate from a solution, you could either use 100.0 mL of a 5.0 M solution, or 250.0 mL of a 2.0 M solution.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the following molecular formulas, determine the empirical formula of each compound: N2O5, PCl3, H2O2, C6H4Cl2.
A) N2O5, PCl3, HO, C6H4Cl2
B) N2O5, PCl3, H2O, C6H4Cl2
C) N2O5, PCl3, H2O2, C3H2Cl2
D) NO2.5, PCl3, HO, C3H2Cl
E) N2O5, PCl3, HO, C3H2Cl
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the following molecular formulas, determine the empirical formula of each compound: Cl2O5, PbCl4, N2O4, C3H6Cl2.
A) ClO2.5, PbCl4, NO2, C3H6Cl2
B) Cl2O5, PbCl4, NO2, C3H6Cl2
C) Cl2O5, PbCl4, NO2, CH2Cl
D) Cl2O5, PbCl4, N2O4, C3H6Cl2
E) Cl2O5, PbCl, N2O4, C3H6Cl2
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the substances in the figure from least atoms per mole to most atoms per mole. 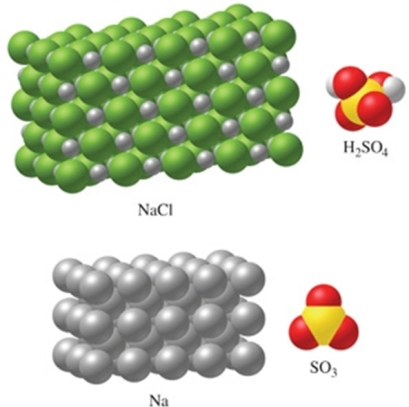
A) SO3 < H2SO4 < NaCl < Na
B) Na < NaCl < SO3 < H2SO4
C) Na < NaCl < H2SO4 < SO3
D) NaCl < Na < SO3 < H2SO4
E) H2SO4 < SO3 < NaCl < Na
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following minerals contain zinc.Rank the minerals in order of percent zinc from least to greatest: sphalerite, ZnS; smithsonite, ZnCO3; zincite, ZnO; gahnite, ZnAl2O4.
A) ZnO < ZnS < ZnAl2O4 < ZnCO3
B) ZnO < ZnS < ZnCO3 < ZnAl2O4
C) ZnAl2O4 < ZnCO3 < ZnS < ZnO
D) ZnAl2O4 < ZnCO3 < ZnO < ZnS
E) ZnCO3 < ZnAl2O4 < ZnS < ZnO
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If you have 10.0 g of sodium chloride, NaCl, how many formula units are in the sodium chloride sample?
A) 2.84 x 1025
B) 3.52 x 1024
C) 6.02 x 1024
D) 1.03 x 1023
E) 0.171
G) A) and E)
Correct Answer

verified
Correct Answer
verified
True/False
Of the substances shown in the figure, only N2O3 has an empirical formula that is the same as its molecular formula. 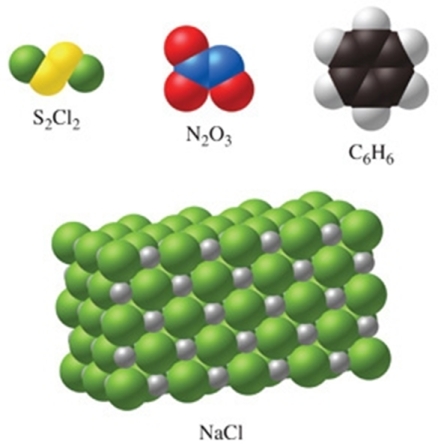
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If the molar mass of a substance is 20.0 g/mol, what is the mass of 3.01 x 1025 molecules of the substance?
A) 0.400 g
B) 10.0 g
C) 1.00 x 103 g
D) 6.02 x 1026 g
E) 1.20 x 1025 g
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the molar mass of CuSO4.5H2O.
A) 159.62 g/mol
B) 249.70 g/mol
C) 177.64 g/mol
D) 185.72 g/mol
E) 446.48 g/mol
G) A) and B)
Correct Answer

verified
Correct Answer
verified
True/False
The solute in the solution shown in the figure is CaCl. 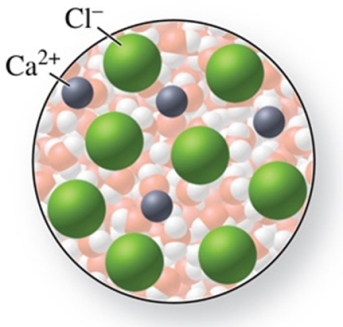
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In order to prepare a 0.0500 M NaOH solution, to what volume would you dilute 25.0 mL of 2.50 M NaOH?
A) 1.25 x 103 L
B) 1.25 L
C) 1.25 mL
D) 0.0625 L
E) 0.500 L
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Showing 81 - 100 of 111
Related Exams