A) CH3CH=CHCH3 + 2 H2 → 2 CH3CH3
B) CH3CH=CHCH3 + 3 H2 → CH3CH3 + 2 CH4
C) CH3CH=CHCH3 + 2 H2 → CH3CH2CH3 + CH4
D) CH3CH=CHCH3 + H2 → CH3CH2CH2CH3
E) CH3CH=CHCH3 + 4 H2 → 4 CH4
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Write the balanced chemical equation for the addition of 2 Br2 to CH3C≡CH.
A) CH3C≡CH + 2 Br2 → CH3CBr2CHBr2
B) CH3C≡CH + 2 Br2 → CH3CBr=CHBr
C) CH3C≡CH + 2 Br2 → CH3CH2CHBr2
D) CH3C≡CH + 2 Br2 → CH3CBr2CH3
E) CH3C≡CH + 2 Br2 → CH3CHBrCH2Br
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The difference between ethane and ethylamine is the presence of which element?
A) carbon
B) oxygen
C) hydrogen
D) nitrogen
E) sulfur
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Name the following compound. 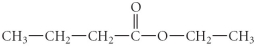
A) ethyl pentyl ether
B) methyl propanoate
C) ethyl butanoate
D) hexanoic acid
E) 4- hexanone
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the weak base from the compounds below.
A) (CH3) 2NH
B) CH3COOH
C) CH3SeCH3
D) CH3CH3
E) CH3CH2Br
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Write the balanced chemical equation that represents the addition of Cl2 to CH3CH=CH2.
A) CH3CH=CH2 + Cl2 → CH3CHClCH3 + HCl
B) CH3CH=CH2 + Cl2 → CH3CCl=CHCl + H2
C) CH3CH=CH2 + Cl2 → CH3CHClCH2Cl
D) CH3CH=CH2 + 2 Cl2 → CH3CHCl2 + CH2Cl2
E) CH3CH=CH2 + 2 Cl2 → CH3CCl2CHCl2 + H2
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Name the following compound. 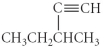
A) 3-methyl-1-pentyne
B) 3-methyl-4-pentyne
C) 2-ethynebutane
D) 1-hexyne
E) 3-ethyl-1-butyne
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Arrange the following in order from least oxidized to most oxidized. 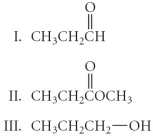
A) II < I < III
B) III < I = II
C) III < I < II
D) I < II < III
E) I < III < II
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following molecules is the most polar?
A) butane
B) butanoic acid
C) cyclohexane
D) ethanol
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Name the following compound. 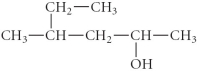
A) 3-methyl-5-hexanol
B) 2- heptenol
C) 4-methyl-2-hexanol
D) 2-ethyl-4- pentenol
E) 4-ethyl-2- pentenol
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What compound is combined with yeast to form ethanol?
A) glucose
B) methanol
C) propanone
D) naphthalene
E) ethane
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Name the following compound. 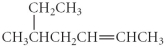
A) 2-ethyl-4- hexene
B) 4-isobutyl-2- butene
C) 3-methyl-5- heptane
D) 4-isopropyl-2- butene
E) 5-methyl-2-heptene
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Complete and balance the following complete hydrogenation reaction. CH3CH2C  CCH3 + 2 H2 → ?
CCH3 + 2 H2 → ?
A) CH3CH2C ![]() CCH3 + 3 H2 → CH3CH2CH3 + CH3CH3
CCH3 + 3 H2 → CH3CH2CH3 + CH3CH3
B) CH3CH2C ![]() CCH3 + 2 H2 → CH3CH2CH2CH2CH3
CCH3 + 2 H2 → CH3CH2CH2CH2CH3
C) CH3CH2C ![]() CCH3 + 6 H2 → 5 CH4
CCH3 + 6 H2 → 5 CH4
D) 2 CH3CH2C ![]() CCH3 + 2 H2 → CH3CH2CH2CH2CH2CH2CH2CH2CH2CH3
CCH3 + 2 H2 → CH3CH2CH2CH2CH2CH2CH2CH2CH2CH3
E) CH3CH2C ![]() CCH3 + 2 H2 → CH3CH2CH2
CCH3 + 2 H2 → CH3CH2CH2 ![]() CH2CH3
CH2CH3
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the weak acid from the compounds below.
A) CH3CH2NH2
B) CH3CH2COOH
C) CH3CH2SCH3
D) CH3CH2Cl
E) CH3OH
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Name the following compound. 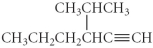
A) 4-propyl-5-hexyne
B) 3-isopropyl-1-hexyne
C) 1-nonyne
D) 4-methyl-3-propyl-1-pentyne
E) 2-methyl-4-pentyne
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Molecules with the same formula but different structures are called
A) structural isomers.
B) achiral.
C) diastereomers.
D) enantiomers.
E) racemic mixture.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is an amine?
A) (CH3CH2) 2NH
B) CH3CH2CH2CH2CO2CH3
C) CH3CH2CH2CH2F
D) CH3CH=O
E) CH3SCH3
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the organic product for the following reaction. CH3CH2CH2NH2 + HCl →
A) ClCH2CH2CH2NH2
B) CH3CHClCH2NH2
C) CH3CH2CHClNH2
D) CH3CH2CH2NHCl
E) CH3CH2CH2NH3+ Cl-
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following names is correct?
A) 1,2,4-triiodobenzene
B) 1- ethyl-3-isopropylhexene
C) 1,3,4- chlorobenzene
D) 4-isopropyl-6-methylbenzene
E) 2,5-dibromocyclohexene
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Name the following compound. 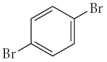
A) o-dibromobenzene
B) m-dibromobenzene
C) p-dibromobenzene
D) p-bromobenzene
E) none of the above
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Showing 81 - 100 of 104
Related Exams