A) 5
B) 7
C) 3
D) 2
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which has the highest meting point?
A) NaCl
B) KCl
C) RbCl
D) CsCl
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Using periodic trends,place the following bonds in order of decreasing ionic character. Sb-Cl P-Cl As-Cl
A) Sb-Cl > As-Cl > P-Cl
B) As-Cl > Sb-Cl > P-Cl
C) Sb-Cl > P-Cl > As-Cl
D) P-Cl > As-Cl > Sb-Cl
E) P-Cl > Sb-Cl > As-Cl
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following represent the Lewis structure for Cl?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following ionic compounds would be expected to have the highest lattice energy?
A) LiF
B) LiCl
C) LiBr
D) LiI
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the bond below that is most polar.
A) H-I
B) H-Br
C) H-F
D) H-Cl
E) C-H
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following represent the Lewis structure for N?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the compound below that should have the lowest melting point according to the ionic bonding model.
A) LiF
B) NaCl
C) CsI
D) KBr
E) RbI
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
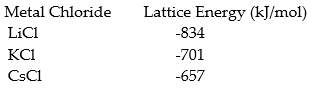 Using the data predict the lattice energy of NaCl
Using the data predict the lattice energy of NaCl
A) -787 kJ/mol
B) -900 kJ/mol
C) -680 kJ/mol
D) -600 kJ/mol
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which ionic compound would be expected to have the highest lattice energy?
A) Rb2O
B) SrO
C) In2O3
D) CO2
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which molecule or compound below contains a polar covalent bond?
A) C2H4
B) ZnS
C) LiI
D) NCl3
E) AgCl
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Draw the Lewis structure for NO2⁻ including any valid resonance structures.Which of the following statements is true?
A) The nitrite ion contains one N-O single bond and one N=O double bond.
B) The nitrite ion contains two N-O bonds that are equivalent to 1.5 bonds.
C) The nitrite ion contains two N=O double bonds.
D) The nitrite ion contains two N-O single bonds.
E) None of the above are true.
G) All of the above
Correct Answer

verified
Correct Answer
verified
Essay
Describe the difference between a pure covalent bond and a polar covalent bond.
Correct Answer

verified
A pure covalent bond occurs when bonding...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which of the following processes is exothermic?
A) the second ionization energy of Mg
B) the sublimation of Li
C) the breaking the bond of I2
D) the formation of NaBr from its constituent elements in their standard state
E) None of the above are exothermic.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following reactions is associated with the lattice energy of CaS (ΔH°latt) ?
A) Ca(s) + S(s) → CaS(s)
B) CaS(s) → Ca(s) + S(s)
C) Ca2⁺(aq) + S2⁻(aq) → CaS(s)
D) Ca2⁺(g) + S2⁻(g) → CaS(s)
E) CaS(s) → Ca2+(aq) + S2⁻(aq)
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the best Lewis structure for SO42⁻.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Using Lewis structures and formal charge,which of the following ions is most stable? OCN⁻ ONC⁻ NOC⁻
A) OCN⁻
B) ONC⁻
C) NOC⁻
D) None of these ions are stable according to Lewis theory.
E) All of these compounds are equally stable according to Lewis theory.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the formula of an ionic compound between aluminum and oxygen.
A) AlO2
B) AlO
C) Al2O3
D) Al3O2
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Short Answer
Describe a covalent bond.
Correct Answer

verified
A bond for...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Choose the best Lewis structure for BF3.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Showing 81 - 100 of 125
Related Exams