A) C2H4
B) CCl4
C) H2O
D) N2
E) O2
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement properly describes the formal charges on the atoms in 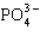 ?
?
A) -2 on oxygen, +5 on phosphorus
B) -1 on oxygen, +4 on phosphorus
C) -1 on oxygen, +1 on phosphorus
D) +1 on oxygen, -1 on phosphorus
E) +1 on oxygen, -3 on phosphorus
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the number of electrons required by these four elements, in the order listed, to achieve an octet of electrons. 
A) 1 3 2 4
B) 3 4 2 2
C) 3 2 4 1
D) 4 3 5 2
E) 5 6 4 7
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the formal charge on sulfur in SO2?
A) +2
B) +1
C) 0
D) -1
E) -2
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which bond is longest?
A) C-O
B) C-I
C) C-Br
D) C-C
E) C-N
G) A) and B)
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Write the correct Lewis dot structure for O2. Which statement correctly describes the structure of the molecule?
A) There is one double bond and four lone electron pairs.
B) There is one double bond and six lone electron pairs.
C) There is one single bond and four lone electron pairs.
D) There is one single bond and six lone electron pairs.
E) There is one single bond, one double bond, and six lone electron pairs.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
List these compounds in order of decreasing number of valence electrons: CO2, CH3Cl, HCN.
A) CH3Cl, HCN, CO2
B) CO2, HCN, CH3Cl
C) CH3Cl, CO2, HCN
D) CO2, CH3Cl, HCN
E) HCN, CO2, CH3Cl
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many electrons will be in the correctly drawn Lewis Structure for CCl4?
A) 32
B) 74
C) 35
D) 8
E) 11
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many electrons will be in the correctly drawn Lewis Structure for 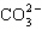 ?
?
A) 20
B) 22
C) 24
D) 26
E) 30
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Short Answer
Benzene is an example of a resonance hybrid because it contains _____________ electrons.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Write the correct Lewis dot structures for the compounds given below. Arrange them in order of shortest to longest bond lengths. Consider only the carbon-carbon and carbon-oxygen bonds. 
A) C2H6 < C2H4 < C2H2 < CO
B) C2H2 < CO < C2H4 < C2H6
C) C2H2 < C2H4 < C2H6 < CO
D) CO < C2H2 < C2H4 < C2H6
E) CO < C2H6 < C2H4 < C2H2
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which bond is least polar?
A) C-C
B) C-N
C) N-H
D) C-F
E) C-O
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compound can exhibit cis-trans isomerism?
A) CH2=CH2
B) CH3CH3
C) H2C=O
D) ClHC=CHCl
E) Cl2C=CH2
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Write the correct Lewis dot structure for CCl2O. Which statement correctly describes the structure?
A) The structure contains 3 single bonds, 1 double bond, and 2 lone electron pairs.
B) The structure contains 3 single bonds, 1 triple bond, and 8 lone electron pairs.
C) The structure contains 2 single bonds, 1 double bond, and 2 lone electron pairs.
D) The structure contains 2 single bonds, 1 double bond and 8 lone electron pairs.
E) The structure contains 1 single bond, 1 triple bond, and 2 lone electron pairs.
G) C) and D)
Correct Answer

verified
D
Correct Answer
verified
Short Answer
The ____________ of an element is its ability to pull electrons towards itself when participating in a covalent bond.
Correct Answer

verified
electronegativity
Correct Answer
verified
Multiple Choice
Which statement about cis and trans isomers is false? The cis- and trans- isomers of a compound:
A) Have the same chemical formulas
B) Have the same molecular weights.
C) Occur in compounds containing triple bonds.
D) Have different boiling points.
E) Have different melting points.
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
From the data below, calculate the approximate enthalpy change for the reaction below. 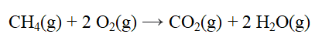

A) -806 kJ
B) -98 kJ
C) 98 kJ
D) 120 kJ
E) 806 kJ
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict qualitatively the relative bond lengths of the four single bonds given below and arrange them from shortest to longest: 
A) C-C < N-N < O-O < F-F
B) N-N < O-O < F-F < C-C
C) F-F < O-O < N-N < C-C
D) O-O < N-N < F-F < C-C
E) C-C < F-F < N-N < O-O
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Assume all hydrocarbons given are linear. Which compound will contain no multiple bonds?
A) C2H4
B) C3H4
C) C6H14
D) C8H16
E) C9H18
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Write the singly bonded Lewis dot structure for BF3. Which statement best describes this structure?
A) The octet rule is obeyed for all atoms.
B) At least one atom has less than an octet of electrons.
C) There is a lone pair of electrons on the boron atom.
D) The boron atom has a formal charge of +1.
E) At least one atom exceeds the octet rule.
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 67
Related Exams