A) 8.400
B) 5.600
C) 9.000
D) 3.980
E) 7.000
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the pOH of an aqueous solution at 25.0°C in which [H+] is 0.0025 M?
A) 8.01
B) 11.40
C) -11.40
D) -8.01
E) none of the above
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Using the data in the table, which of the conjugate bases below is the strongest base? 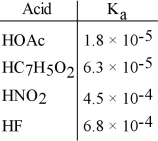
A) OAc-
B) C7H5O2-
C) NO2-
D) F-
E) OAc- and C7H5O2-
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Of the following substances, an aqueous solution of __________ will form basic solutions. NH4Cl Cu(NO3) 2 K2CO3 NaF
A) NH4Cl, Cu(NO3) 2
B) K2CO3, NH4Cl
C) NaF only
D) NaF, K2CO3
E) NH4Cl only
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Of the following substances, an aqueous solution of __________ will form basic solutions. NaHS Cu(NO3) 2 KHCO3 NaF
A) NaHS, Cu(NO3) 2
B) KHCO3, NaHS
C) NaF only
D) NaF, KHCO3
E) NaHS, KHCO3 and NaF
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the concentration (in M) of hydroxide ions in a solution at 25.0°C with a pOH of 4.223.
A) 5.98 × 10-5
B) 1.67 × 10-10
C) 1.67 × 104
D) 5.99 × 10-19
E) 1.00 × 10-7
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the pH of a solution at 25.0 °C that contains 1.94 × 10-10 M hydronium ions.
A) 1.94
B) 4.29
C) 7.00
D) 14.0
E) 9.71
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the pOH of an aqueous solution at 25.0 °C in which [OH-] is 0.0040 M?
A) 11.60
B) -2.40
C) 2.40
D) -11.60
E) 5.52
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Ammonia is a __________.
A) weak acid
B) strong base
C) weak base
D) strong acid
E) salt
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which solution below has the highest concentration of hydroxide ions?
A) pH = 3.21
B) pH = 12.6
C) pH = 7.93
D) pH = 9.82
E) pH = 7.00
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Kb for NH3 is 1.8 × 10-5. What is the pH of a 0.45 M aqueous solution of NH4Cl at 25.0 °C?
A) 2.55
B) 11.45
C) 9.20
D) 4.80
E) 11.23
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The molar concentration of hydronium ion in pure water at 25°C is __________.
A) 0.00
B) 1.0 × 10-7
C) 1.0 × 10-14
D) 1.00
E) 7.00
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the concentration (in M) of hydronium ions in a solution at 25.0 °C with pH = 4.153?
A) 4.15
B) 9.85
C) 1.42 × 10-10
D) 7.03 × 10-5
E) none of the above
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the pOH of 0.716 M anilinium hydrochloride (C6H5NH3Cl) solution in water, given that Kb for aniline is 3.83 × 10-4.
A) 1.78
B) 12.22
C) 5.37
D) 8.63
E) 12.42
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The base-dissociation constant, Kb, for pyridine, C5H5N, is 1.4 × 10-9. The acid-dissociation constant, Ka, for the pyridinium ion, C5H5NH+, is __________.
A) 1.0 × 10-7
B) 1.4 × 10-23
C) 7.1 × 10-4
D) 1.4 × 10-5
E) 7.1 × 10-6
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the pOH of an aqueous solution at 25.0 °C that contains 3.98 × 10-9 M hydroxide ion?
A) 8.40
B) 5.60
C) 9.00
D) 3.98
E) 7.00
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following statements regarding Kw is false?
A) pKw is 14.00 at 25°C
B) The value of Kw is always 1.0 × 10-14.
C) Kw changes with temperature.
D) The value of Kw shows that water is a weak acid.
E) Kw is known as the ion product of water.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The conjugate acid of CH3NH2 is __________.
A) CH3NH2
B) CH3NH3+
C) CH3NH3+
D) CH3NH+
E) none of the above
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The Ka of hydrazoic acid (HN3) is 1.9 × 10-5 at 25.0°C. What is the pH of a 0.40 M aqueous solution of HN3?
A) 0.40
B) 2.16
C) 5.23
D) 2.56
E) -3.46
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The Ka for HCN is 4.9 × 10-10. What is the value of Kb for CN-?
A) 2.0 × 10-5
B) 4.0 × 10-6
C) 4.9 × 104
D) 4.9 × 10-24
E) 2.0 × 109
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Showing 81 - 100 of 134
Related Exams