Correct Answer

verified
Correct Answer
verified
Essay
Provide the structure of the major organic product which results in the following reaction. 
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following alkyl bromides undergoes solvolysis in methanol without rearrangement?
A) (R) -2-bromo-3-ethylpentane
B) (S) -2-bromo-3-ethylpentane
C) (R) -3-bromo-2-methylpentane
D) (S) -3-bromo-2-methylpentane
E) 3-bromo-3-ethylpentane
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the best nucleophile in water?
A) I-
B) CH3SCH3
C) CH3OCH3
D) Cl-
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the alkyl halide that reacts the fastest in a E1 reaction.
A) ![]()
B) CH2 ![]() CHCl
CHCl
C) ![]()
D) ![]()
E) CH3CH2CH2Cl
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is not true for a SN1 mechanism?
A) The rate is dependent on the nucleophile.
B) The rate is fastest in highly polar solvents.
C) proceeds with a carbocation intermediate
D) is a two step reaction
E) Methyl iodide will not undergo this reaction.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Essay
Draw all likely alkene products in the following reaction and circle the product you expect to predominate. 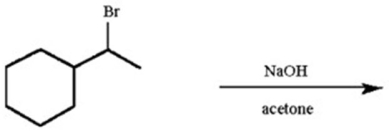
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the halide(s) that react in a SN1 reaction.You may choose more than one answer.
A) benzyl bromide
B) bromobenzene
C) 1-bromo-1-butene
D) 1-bromo-2-butene
E) 2-bromo-2-phenylpropane
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which alkyl halide reacts the fastest in an E2 reaction?
A) 2-chloro-2-methylbutane
B) 1-chlorobutane
C) 1-chloro-2-methylbutane
D) 2-chlorobutane
E) chloromethane
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Essay
List the following compounds in order of increasing reactivity in an SN1 reaction. CH3Br,CH3CH2CH2I,(CH3)3CI,CH3CHBrCH3,CH3CHICH3
Correct Answer

verified
CH3Br < CH3C...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
How would you obtain the following products from 1-bromo-1-methylcyclohexane? 
Correct Answer

verified
1.(CH3)3COK ...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
What is the leaving group in the reaction shown below? 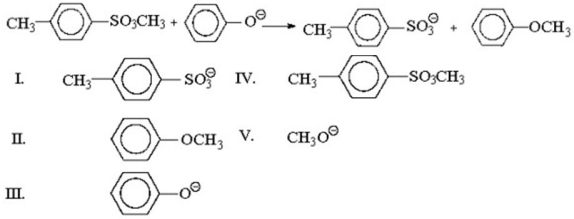
A) I
B) II
C) III
D) IV
E) V
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Essay
Provide the major organic product(s)of the reaction shown. 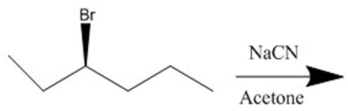
Correct Answer

verified
Correct Answer
verified
Essay
Provide the major organic products(s)of the reaction shown. 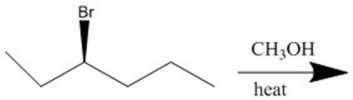
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which halide reacts most rapidly via an SN2 mechanism?
A) (CH3) CCH2CH2F
B) (CH3) CCH2CH2Cl
C) (CH3) CCH2CH2Br
D) (CH3) CCH2CH2I
E) All primary halides react at the same rate in SN2.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the major product of the following reaction? 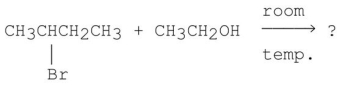
A) CH2 ![]() CHCH2CH3
CHCH2CH3
B) CH3CH ![]() CHCH3
CHCH3
C) ![]()
D) CH3CH2CH2CH2OCH2CH3
E) ![]()
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Essay
What would be the product of this reaction? Also,provide the name of the mechanism (SN2,SN1,E2 or E1). 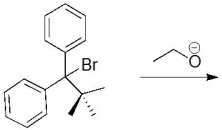
Correct Answer

verified
No reaction.It should undergo ...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which of the following compounds undergoes E2 reactions with the fastest rate?
A) ![]()
B) CH3CH2CH2Cl
C) CH3CH2CH2I
D) ![]()
E) CH3CH2CH2Br
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following SN2 reactions is the slowest?
A) CH3CH2CHBrCH3 + OH- → CH3CH2CHOHCH3 + Br-
B) CH3CH2CHBrCH3 + H2O → CH3CH2CHOHCH3 + HBr
C) CH3CH2CH2CH2Br + OH- → CH3CH2CH2CH2OH + Br-
D) CH3CH2CH2CH2Br + H2O → CH3CH2CH2CH2OH + HBr
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following alkyl halides gives the fastest SN1 reaction?
A) CH3CH2CH2Br
B) ![]()
C) ![]()
D) CH3CH2CH2I
E) CH3CH2CH2Cl
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Showing 161 - 180 of 228
Related Exams